前列腺癌(PCa)是最常见的恶性肿瘤,影响全球超过14%的男性,并占男性癌症相关死亡的约1/14[1]。转移性前列腺癌(PCa)至今仍缺乏有效治疗手段,临床需求巨大,亟待新的药物靶点与治疗策略。细胞膜蛋白STEAP1(six-transmembrane epithelial antigen of prostate 1)在超过85%的前列腺肿瘤中呈高表达,而正常组织几乎不表达,使其成为极具潜力的精准靶向分子[2]。
近岸蛋白全新上线STEAP1靶点蛋白产品,依托公司专业的多次跨膜蛋白表达与纯化平台,提供去垢剂和VLP形式的高质量六次跨膜全长STEAP1抗原,均由人源细胞表达,并经ELISA和BLI验证具备良好生物活性,适用于抗体免疫、筛选及质量控制等关键环节,助力新靶点抗体药物研发高效推进。
STEAP1简介
STEAP1属于STEAP蛋白家族[3],该家族包含STEAP1-4四个成员,均为具有六个跨膜结构域、细胞质N端的多跨膜蛋白。STEAP1作为金属还原酶,可将铁离子还原,支持细胞代谢与增殖。与STEAP2-4不同,STEAP1缺乏细胞质氧化还原酶结构域[4],但其分子构型可形成同源三聚体或与其他STEAP蛋白形成异源三聚体,从而增强其活性并可能影响细胞间通讯。
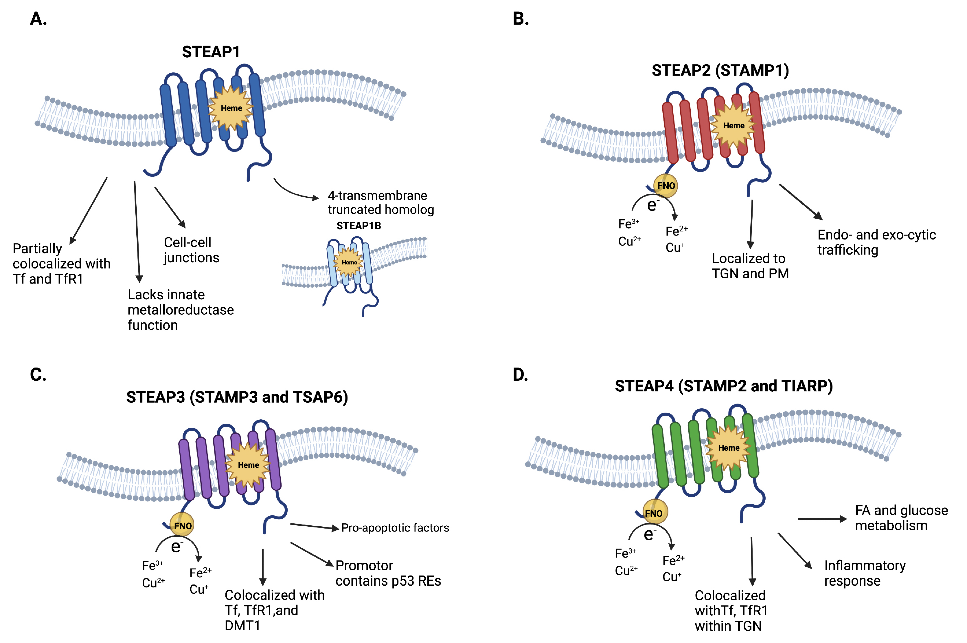
图1. STEAP靶点蛋白的结构[5]
STEAP1的五大治疗策略[2]
STEAP1已成为PCa治疗的有效靶点,相关策略正在积极探索(表1)。目前针对STEAP1的方法包括T细胞双特异抗体、嵌合抗原受体(CAR)T细胞疗法、抗体-药物偶联物(ADC)以及癌症疫苗等。这些疗法旨在直接清除表达STEAP1的肿瘤细胞或动员免疫细胞增强抗肿瘤反应(图2)。

表1. STEAP1的五大治疗策略[2]
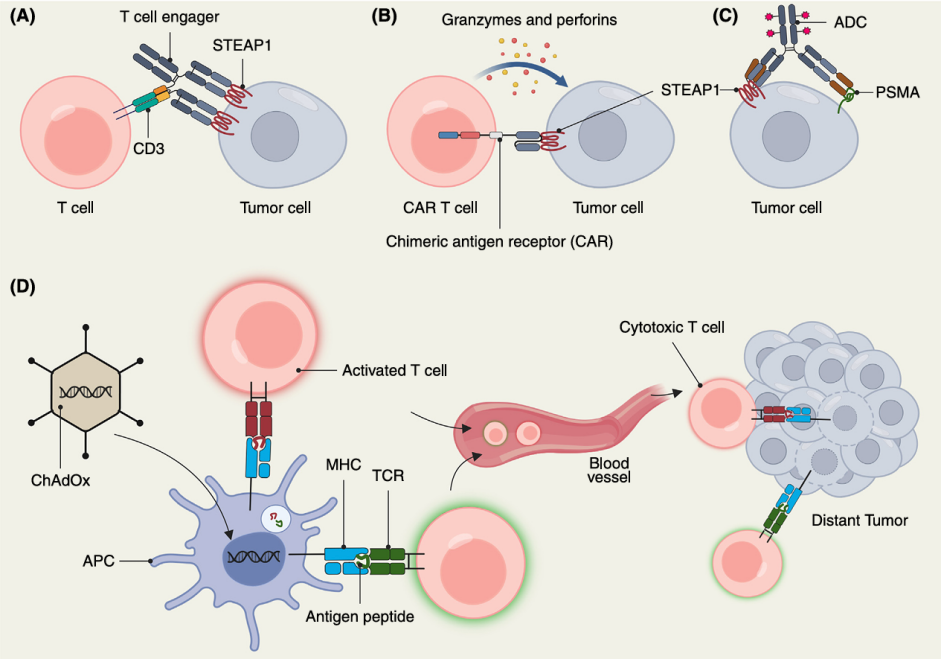
图2. 靶向STEAP1高表达肿瘤的多种免疫治疗策略[2]
靶向STEAP1药物在研情况
AMG-509是安进开发的STEAP1/CD3双特异性抗体,在转移性去势抵抗性前列腺癌(mCRPC)患者中展现出良好疗效:PSA50达49%,ORR达41%,DCR达79%,肿瘤缩小明显,安全性可控。随着三期临床启动,AMG 509有望成为前列腺癌免疫治疗的新突破口。截至2025年7月9日,药渡数据库显示国外已有10家公司围绕STEAP1靶点开展不同阶段的临床研究,国内也有3家相关研发管线。
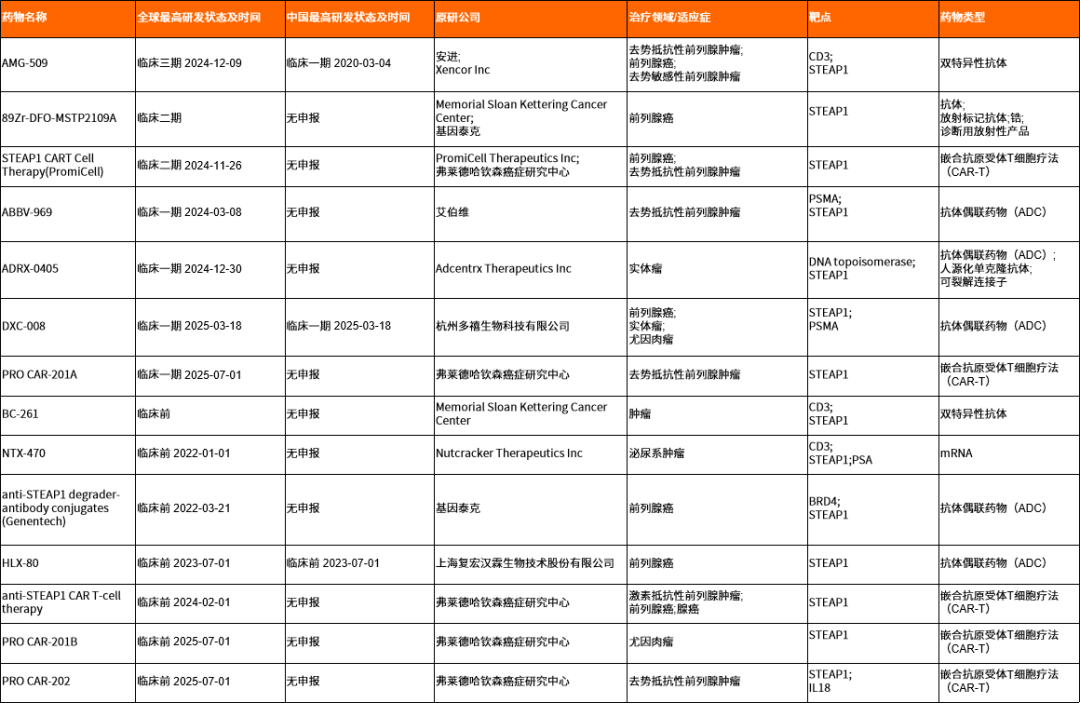
表2. 靶向STEAP1药物进展
基于专业的多次跨膜蛋白技术平台,近岸蛋白开发出高质量全长STEAP1蛋白,具有正确的天然构象和稳定的生物学活性,是STEAP1靶向药物开发重要的蛋白工具。
部分数据展示
ELISA验证
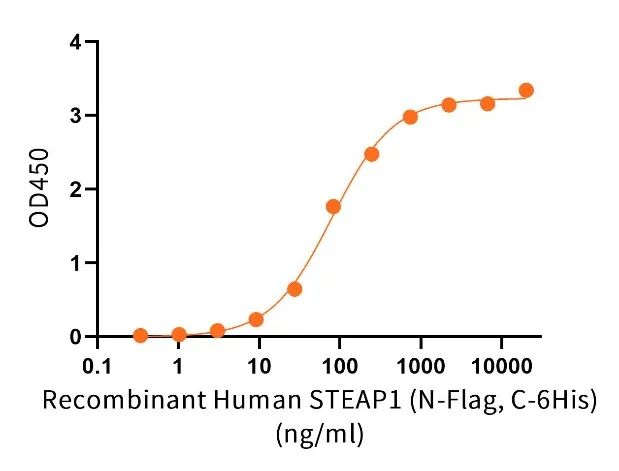
Immobilized Anti-Human STEAP1 Antibody (VAN_bio, Research Grade) (Cat#NC338) at 2μg/ml (100 μl/well) can bind Recombinant Human STEAP1 (N-Flag, C-6His) (Cat#C48A-A).The EC50 of Recombinant Human STEAP1 (N-Flag, C-6His)(Cat#C48A-A) is 79.33ng/ml.
BLI验证
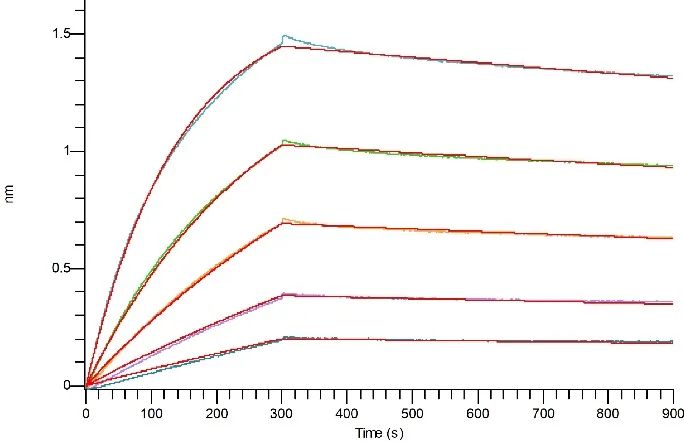
Loaded Anti-Human STEAP1 Antibody (VAN_bio, Research Grade) (Cat#NC338) on Pro-A Biosensor, can bind Recombinant Human STEAP1 (N-Flag, C-6His) (Cat#C48A-A) with an affinity constant of 5.89 nM as determined in BLI assay.
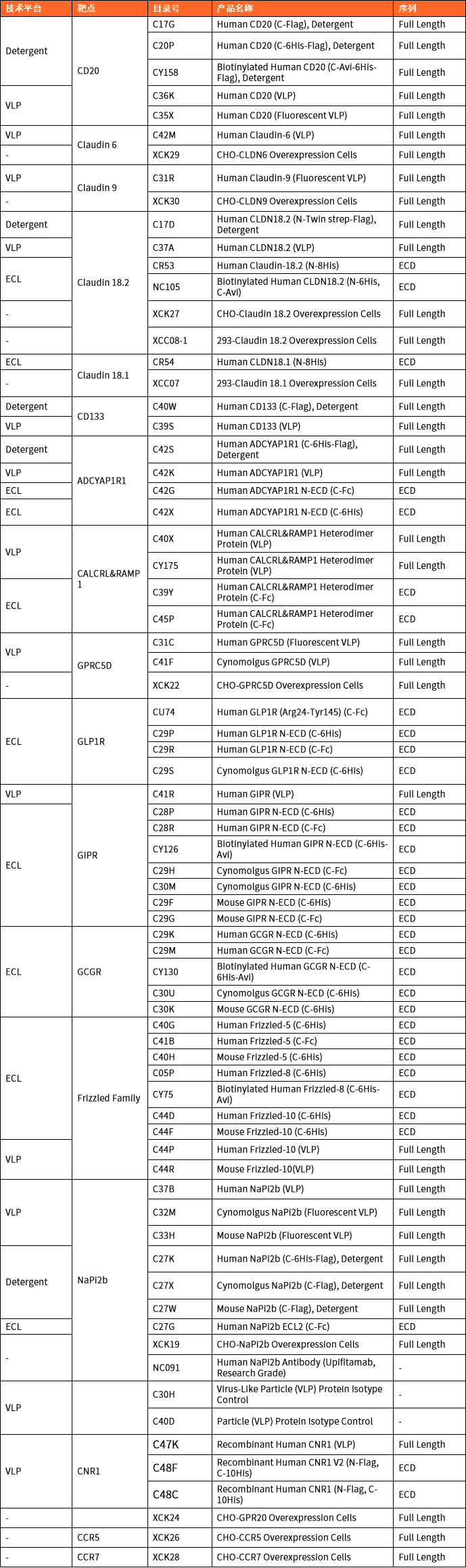
产品推荐
|
|
|
|
|
|
|
|
Recombinant Human STEAP1(N-Twin-Strep, C-10His) |
|
|
|
|
|
|
|
|
|
|
|
|
多次跨膜蛋白相关产品
参考文献
[1]Bray, Freddie, et al. "Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries." CA: a cancer journal for clinicians 74.3 (2024): 229-263. DOI: 10.3322/caac.21834.
[2]Singh, Rohit, and Jon Amund Kyte. "STEAP1: a promising target in prostate cancer therapy." Trends in Cancer (2025). DOI: 10.1016/j.trecan.2025.05.007.
[3]Chen, Wen-Jia, et al. "Regulatory roles of six-transmembrane epithelial antigen of the prostate family members in the occurrence and development of malignant tumors." Frontiers in Cell and Developmental Biology 9 (2021): 752426. DOI: 10.3389/fcell.2021.752426.
[4]Oosterheert, Wout, and Piet Gros. "Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1)." Journal of Biological Chemistry 295.28 (2020): 9502-9512. DOI: 10.1074/jbc.RA120.013690.
[5]Xu, Michael, et al. "STEAP1–4 (six-transmembrane epithelial antigen of the prostate 1–4) and their clinical implications for prostate cancer." Cancers 14.16 (2022): 4034. DOI: 10.3390/cancers14164034.
[6]Nolan-Stevaux, Olivier, et al. "AMG 509 (xaluritamig), an anti-STEAP1 XmAb 2+ 1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer." Cancer Discovery 14.1 (2024): 90-103. DOI: 10.1158/2159-8290.CD-23-0984.
[7]Kelly, William K., et al. "Xaluritamig, a STEAP1× CD3 XmAb 2+ 1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study." Cancer discovery 14.1 (2024): 76-89. DOI: 10.1158/2159-8290.CD-23-0964.
[8]Bhatia, Vipul, et al. "Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy." Nature Communications 14.1 (2023): 2041. DOI: 10.1038/s41467-023-37874-2.
[9]Bizzaro, Candice L., et al. "Exploring STEAP1 expression in prostate cancer cells in response to androgen deprivation and in small extracellular vesicles." Molecular Cancer Research 23.6 (2025): 542-552. DOI: 10.1158/1541-7786.MCR-24-0903.
[10]Berger, R., et al. "1660TiP First-in-human study of ABBV-969, a dual variable antibody-drug conjugate (ADC), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC)." Annals of Oncology 35 (2024): S1000-S1001. DOI: 10.1016/j.annonc.2024.08.1741.
[11]Danila, Daniel C., et al. "Phase I study of DSTP3086S, an antibody-drug conjugate targeting six-transmembrane epithelial antigen of prostate 1, in metastatic castration-resistant prostate cancer." Journal of Clinical Oncology 37.36 (2019): 3518-3527. DOI: 10.1200/JCO.19.00646.
[12]Stein, Mark N., et al. "Prime-boost immunotherapeutic trial in men with biochemical recurrence after definitive local therapy for prostate cancer." (2023): TPS5119-TPS5119. DOI:10.1200/JCO.2023.41.16_suppl.TPS5119.
[13]Carrasquillo, Jorge A., et al. "Imaging patients with metastatic castration-resistant prostate cancer using 89Zr-DFO-MSTP2109A anti-STEAP1 antibody." Journal of Nuclear Medicine 60.11 (2019): 1517-1523. DOI: 10.2967/jnumed.118.222844.
